Milada Teplá
KUDCH, PřF UK v Praze
email: milada.tepla@natur.cuni.cz
Biochemie - základní kapitoly
Buňka - úvod
Nukleové kyseliny a proteosyntéza
Přírodní látky
Trávení
Metabolismus
- Základní pojmy
- Metabolismus sacharidů
- Metabolismus triacylglycerolů
- Metabolismus bílkovin
- Citrátový cyklus
- Vztahy mezi metabolismy
- Lokalizace pochodů v buňce
- Dýchací řetězec
- Fotosyntéza
- Photosynthesis
- Electron Transport Chain
- Výukové materiály
Další informace
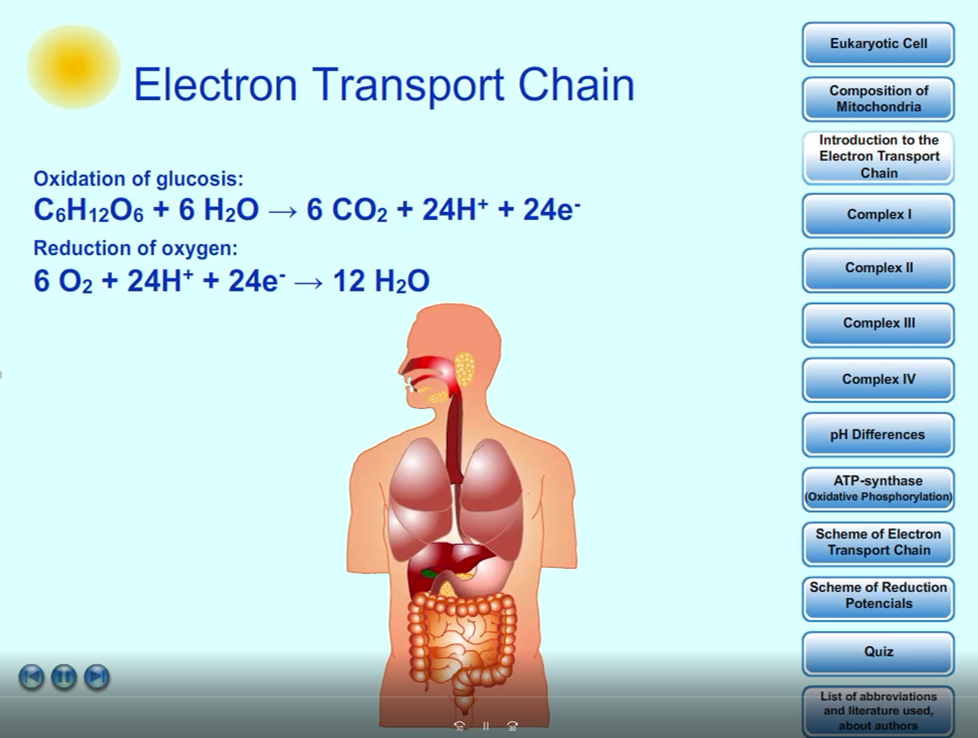
Electron Transport Chain
Education materials:
|
Eukaryotic Cell + Composition of Mitochondria: |
|
|
Chapters:
1. Introduction
2. Eukaryotic Cell
3. Composition of Mitochondria
4. Introduction to the Electron Transport Chain and Coenzymes NADH a FADH2
5. Complex I
6. Complex II
7. Complex III
8. Complex IV
9. ATP-synthase (Oxidative Phosphorylation)
10. Scheme of Reduction Potencials
11. Scheme of Electron Transport Chain
12. Quiz
13. List of Abbreviations
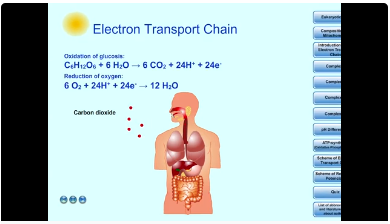
1. Introduction
C6H12O6 + 6 O2 + 6 H2O → 6 CO2 + 12 H2O
2. Eukaryotic Cell
Plant and animal cells are eukaryotic cells and have several differences and similarities. The electron transport chain (= the respiratory chain or cellular respiration) occurs in the mitochondria.
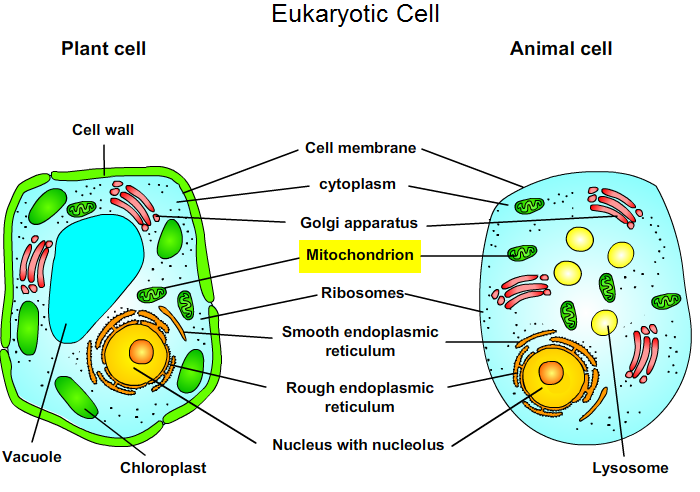 Fig. 1: Eukaryotic Cell.
Fig. 1: Eukaryotic Cell.
3. Composition of Mitochondria
Mitochondria are semiautonomous organelle (contains its own DNA). They have two types of membranes: the outer and inner membrane.The outer mitochondrial membrane contains protein porin that permit free diffusion. The inner membrane is freely permeable only to O2, CO2 and H2O. The inner membrane contains numerous transport proteins that control the passage of most ions, metabolites and low molecular mass compounds. The membranes define two separate areas: the intermembrane space and matrix (a gel like substance of <50% water). The proteins mediating electron transport and oxidative phosphorylation are bound to the inner mitochondrial membrane.
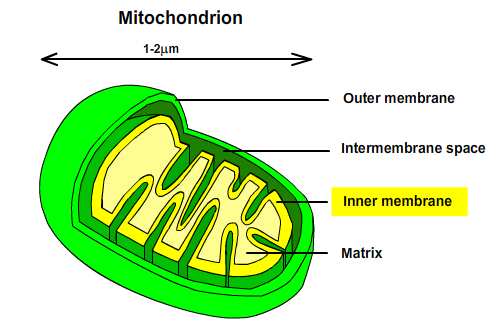
Fig. 2: Composition of Mitochondria.
Eukaryotic Cell + Composition of Mitochondria: movie / https://youtu.be/dfWsjYToIl8
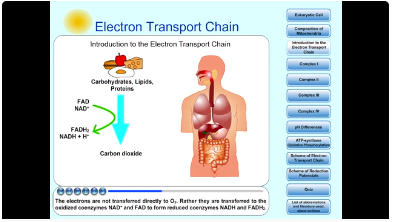
4. Introduction to the Electron Transport Chain and Coenzymes NADH a FADH2
The electron-transport chain is the final and most important step of cellular respiration. This process occurs in the inner membrane of mitochondria. The electron-transport chain is a sequence of redox reactions terminating catabolic degradation of carbohydrates, lipids and proteins. The electrons are not transferred directly to O2. Rather they are transferred to the oxidized coenzymes NAD+ and FAD to form reduced coenzymes NADH and FADH2.
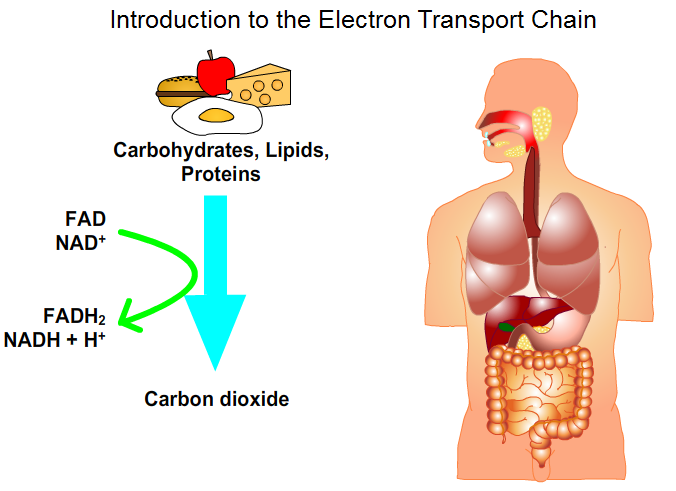
Fig. 3: Introduction to Electron Transport Chain: movie / https://youtu.be/VhUNRkY2ZlU
NADH (nicotinamide adenine dinucleotide) is reoxidized to NAD+ by the electron transport chain:
NADH + H+ → NAD+ + 2 H+ + 2 e-.
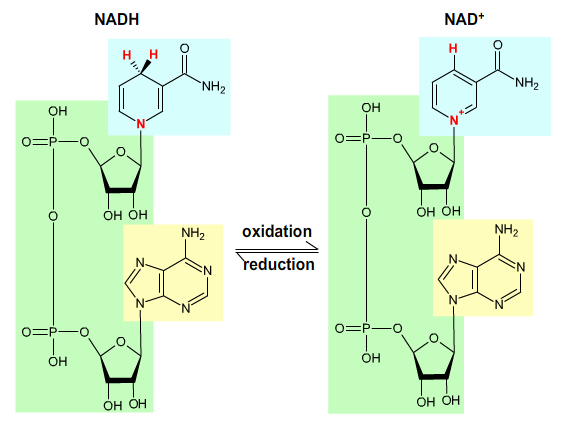
Fig. 4: Nicotinamide adenine dinucleotide.
FADH2 (Flavin adenine dinucleotide) is reoxidized to FAD by the electron transport chain:
FADH2 → FAD + 2 H+ + 2 e-.
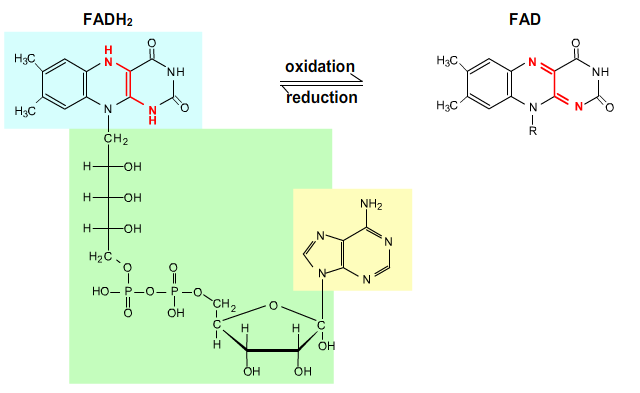
Fig. 5: Flavin adenine dinucleotide.
At the mitochondrial inner membrane, electrons from NADH and FADH2 pass through the electron-transport chain to the final acceptor O2, which is reduced to water. The protons are transferred out of the matrix to the intermembrane space (an electrochemical H+ gradient is created across the inner mitochondrial membrane). The exergonic return of these protons to the mitochondrial matrix powers the synthesis of ATP (adenosine triphosphate).
Complex I (NADH-Coenzyme Q Oxidoreductase) passes electrons from NADH to Coenzym Q (CoQ, ubiquinone), which is reduced to ubiquinol (CoQH2). Oxidation of NADH is strongly favorable reaction (NADH is strong reductant):
- NADH + H+ + CoQ → NAD+ + CoQH2.
Complex I contains one molecule of flavin mononucleotide (FMN) and
iron-sulfur clusters that participate in the electron transport process.
Iron-sulfur clusters contain Fe ions. Fe ions exist in either Fe2+ or Fe3+ oxidation states:
- Fe3+ + e- → Fe2+ → Fe3+ + e-.
Two electrons transfer from NADH to FMN to yield FMNH- and NAD+. The electrons are then transferred, one at a time, to Fe-S clusters and then to CoQ, which is sequentially reduced to ubisemiquinone CoQH• and then to CoQH2. Complex I pumps four protons out of the matrix for every electron pair it translocates from NADH to CoQ. CoQH2 is released into membrane and replaced by CoQ.
Oxidation states of CoQ:
CoQ + H+ + e- → CoQH•
CoQH• + H+ + e- → CoQH2
CoQH2 → CoQH• + H+ + e-
CoQH• → CoQ + H+ + e-
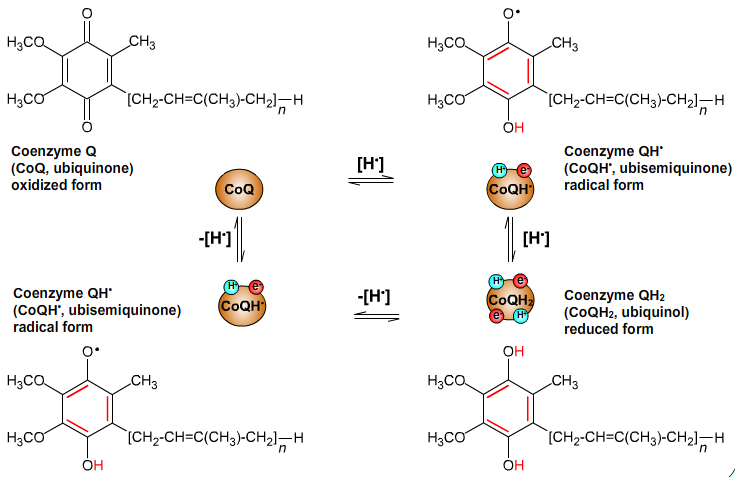
Fig. 6: Oxidation states of CoQ.
6. Complex II
Complex II (Succinate-Coenzyme Q Oxidoreductase) passes electrons from succinate to Coenzym Q (CoQ, ubiquinone) to yield fumarate and ubichinol (CoQH2). Complex II is also the citric acid cycle enzyme succinate dehydrogenase that oxidizes succinate to fumarate. FAD is reduced to FADH2.
Complex II contains cytochrome b (cyt b) and iron-sulfur clusters that participate in the electron transport process. Iron-sulfur clusters and cyt b contain Fe ions. Fe ions exist in either Fe2+ or Fe3+ oxidation states: :
- Fe3+ + e- → Fe2+ → Fe3+ + e-.
The electrons are transferred, one at a time, from FADH2 to Fe-S clusters and then to cyt b. Finally, the electrons are passed to CoQ. Oxidation of FADH2 is favorable reaction (FADH2 is reductant):
- FADH2 + CoQ → FAD + CoQH2
CoQ is sequentially reduced to ubisemiquinone CoQH• and then to CoQH2. CoQH2 is released into membrane and replaced by CoQ.
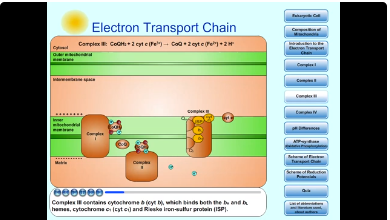
7. Complex III
Complex III (Coenzyme Q-Cytochrome c Oxidoreductase) passes electrons from reduced CoQ (ubiquinol, CoQH2) to cytochrome c.
- CoQH2 + cyt c (Fe3+) → CoQ + cyt c (Fe2+) + 2 H+
Complex III contains cytochrome b (cyt b), which binds both the bH and bL hemes, cytochrome c1 (cyt c1) and Rieske iron-sulfur protein (ISP). Cytochromes and Rieske iron-sulfur protein contain Fe ions. Fe ions exist in either Fe2+ or Fe3+ oxidation states:
- Fe3+ + e- → Fe2+ → Fe3+ + e-.
Complex III pumps protons from the matrix to the intermembrane space via a redox loop mechanism called Q cycle. The essence of the Q cycle is that CoQH2 undergoes a two-cycle reoxidation in which the semiquinone CoQ•- is a stable intermadiate. This involves two independent binding sites for coenzyme Q: Qo, which binds CoQH2; and Qi, which binds CoQ•- and CoQ. CoQH2, which is supplied by Complexes I or II, binds to the Qo site. CoQH2 transfers one of its electrons to the ISP, releasing its two protons into the intermembrane space and yielding CoQ•- . The ISP reduces cyt c1 and then cyt c. CoQ•- reduces heme bL to form CoQ. Heme bL then reduces heme bH. The CoQ is released from the Qo site and diffuses through the membrane to rebind at the Qi site. The CoQ picks up the electron from heme bH, reverting to the semiquinone form, CoQ•- :
- CoQH2 + cyt c1(Fe3+) → CoQ•- + cyt c1(Fe2+) + 2H+ (outside)
Another CoQH2 binds to the Qo site - steps are repeated (One electron reduces the ISP, and the other electron sequentially reduces heme bL and then heme bH. This second electron then reduces the CoQ•- at the Qi site, yielding CoQH2. The protons take up in this last step originate in the mitochondrial matrix. For every two CoQH2 that enter Q cycle, one CoQH2 is regenerated. The overall reaction:
- CoQH2 + 2 cyt c1(Fe3+) + 2H+(matrix) → CoQ + 2 cyt c1(Fe2+) + 4H+ (outside)
8. Complex IV
Complex IV (Cytochrom c Oxidase) catalyzes the one-electron oxidations of four consecutive reduced cytochrome c molecules and the concomitant four-electron reduction of one O2 molecule to yield 2H2O:.
- 4 cyt c (Fe2+) + 02 + 4 H+ → 4 cyt c (Fe3+) + 2 H2O.
Complex IV contains CuA center, cytochrome a (cyt a) and a binuclear complex of cytochrome a3 (cyt a3) and CuB center. Cytochromes contain Fe ions. Fe ions exist in either Fe2+ or Fe3+ oxidation states. Cu-containing centers contain Cu ions. Cu ions exist in either Cu+ or Cu2+ oxidation states.
- Fe3+ + e- → Fe2+ → Fe3+ + e-
- Cu2+ + e- → Cu+ → Cu2++ e-
O2 binds to the binuclear complex of cyt a3 and CuB and is reduced to H2O in a complex four-electron reaction. Cyt c alternately binds to cytochrome c1 (of Complex III) and to Complex IV and thereby functions to shuttle electrons between them. An electron obtained from cyt c is transferred to the CuA center and then to the cyt a. The electron is then transferred to the cyt a3–CuB binuclear complex. O2 molecule is consecutively reduced by four electrons to yield 2H2O:
- 4 H+ + 4 e- + O2 → 2 H2O.
Complex IV must acquire four protons from the inside (matrix) for every molecule of O2 it reduces to H2O. This four-electron process is coupled to the translocation of up to 4H+ from the inside to the outside:
- 4 cyt c (Fe2+) + 02 + 8 H+ (matrix) → 4 cyt c (Fe3+) + 2 H2O + 4 H+ (outside)
Complexes I, II, III, IV + Scheme: movie / https://youtu.be/8Q0hJ1nvgbc
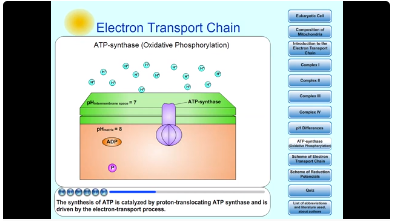
9. ATP-synthase (Oxidative Phosphorylation)
Electron transport causes Complexes I, III and IV to transport protons across the inner mitochondrial membrane from matrix to the intermembrane space. An electrochemical gradient across the inner mitochondrial membrane is created. [H+] in intermembrane space is higher than [H+] in matrix (pH(out) is less than pH(in)). Matrix is a region of low [H+] and negative electrical potential. Intermembrane space is a region of high [H+] and positive electrical potential. The free energy sequestered by the resulting electrochemical gradient (proton-motive force) powers ATP synthesis.
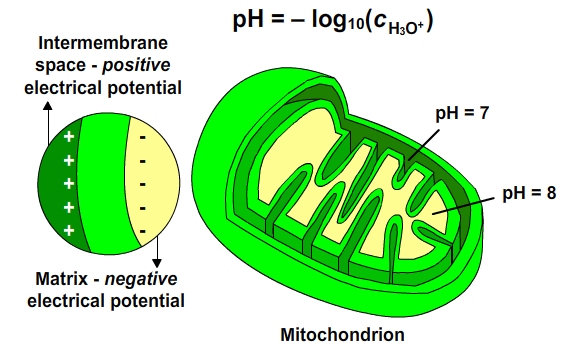
Fig. 7: pH differences.
The synthesis of Adenosine triphosphate (ATP) from Adenosine diphosphate (ADP) and inorganic phosphate (Pi) in mitochondrion is the endergonic process. The synthesis of ATP is catalyzed by proton-translocating ATP synthase and is driven by the electron-transport process. The electrochemical potential of H+ gradient across the inner mitochondrial membrane is harnessed to synthesize ATP. The synthesis of one ATP molecule seems to require the translocation of about three protons. This synthesis of ATP is called oxidative phosphorylation.
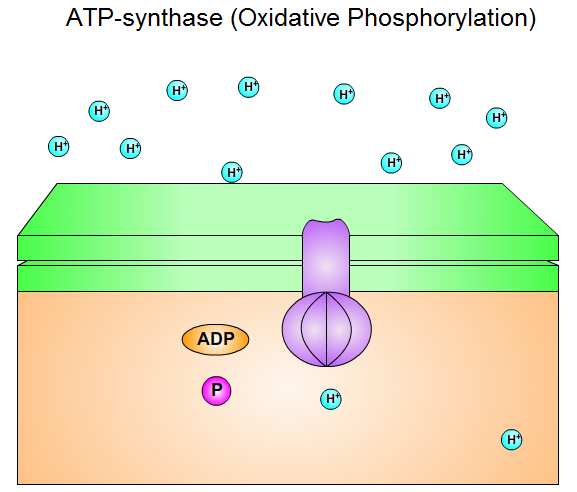
Fig. 8: ATP-synthase (Oxidative Phosphorylation).
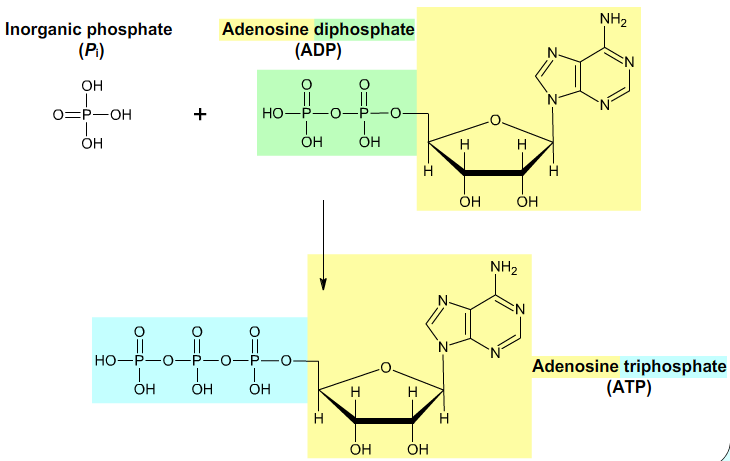
Fig. 9: ATP.
ATP-synthase: movie / https://youtu.be/HC_3nrWdAKI
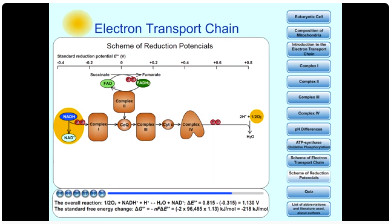
10. Scheme of Reduction Potencials
An oxidized substrate's affinity increases with its standard reduction potential, E°' (1M reactants and products with [H+] defined as 1 at pH 7).Oxygen has higher electron affinity. Oxygen is strong oxidant.
- 1/2O2 + 2H+ + 2e- → H2O; ΔE°' = 0,815 V.
NAD+ has lower electron affinity. NAD+ is weak oxidant; NADH is strong reductant.
- NAD+ + H+ + 2e- → NADH; ΔE°' = - 0,315 V.
NADH is the electron donor in this couple and O2 the electron acceptor. The overall reaction:
- 1/2O2 + NADH+ + H+ ↔ H2O + NAD+; ΔE°' = 0,815 - (-0,315) = 1,130 V
The standard free energy change:: ΔG°' = -nFΔE°' = (-2 x 96 485 x 1,13) kJ/mol = -218 kJ/mol
The standard free energy required to synthesize 1 mol of ATP is 30.5 kJ/mol:
- ADP + Pi → ATP; ΔG°' = 30.5 kJ/mol.
Oxidation of 1 NADH results in the synthesis of ~ 2,5 ATP.
Oxidation of 1 FADH2 results in the synthesis of ~ 1,5 ATP.
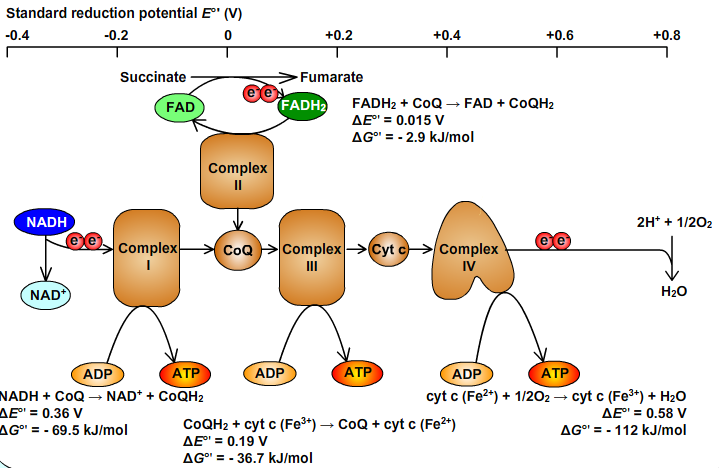
Obr. 10: Scheme of Reduction Potencials.
Scheme of Reduction Potencials: movie / https://youtu.be/M8w_qOIE86U
11. Scheme of Electron Transport Chain
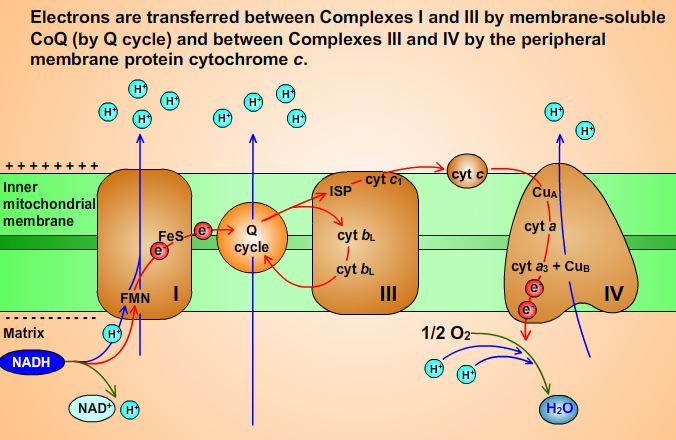 Fig. 11
Fig. 11
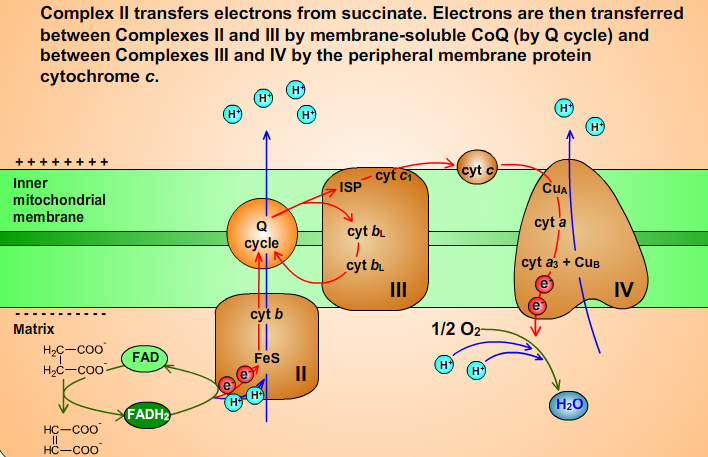 Fig 12.
Fig 12.
12. Quiz

Quiz: movie / https://youtu.be/Jn_T4s0yesE
13. List of Abbreviations
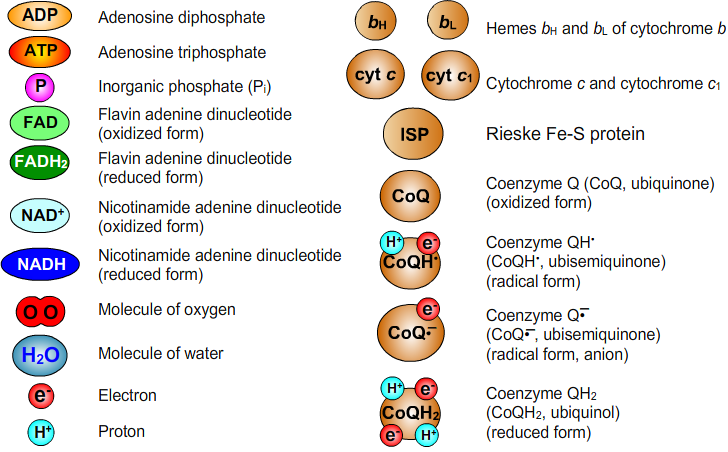
14. Literature
Voet, D. J.; Voet, J. G. Biochemistry, 4th ed.; John Wiley & Sons, Inc.: United States of America, 2011; pp 823-870.